(last update : 15-05-2009)
Tricks we use
Purifying PCR fragments from gel
Sometimes after PCR, you get multiple bands, and you are only interested in one specific band. There are several kits on the marked to purify the band, but all these kits are very expensive. There is a cheaper method to do this :
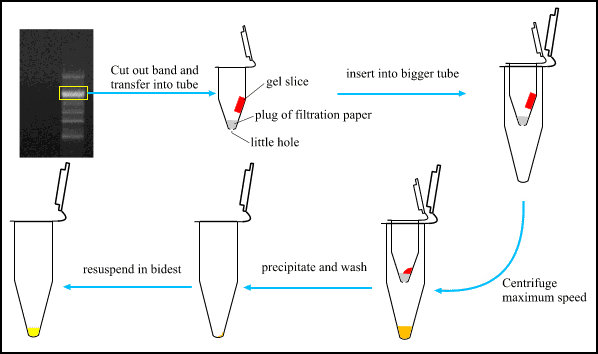
- Separate the fragments on an agarose (or separide) gel.
- Place the gel on an UV source without turning on the UV.
- Remove most of the buffer from the top of the gel with some tissue.
- Turn on the UV-light and cut out the band as soon as possible (do NOT leave the light on longer than necessary !).
- Make a small hole in the bottom of a 0,5 ml tube with a needle, and push a little filtration paper in the bottom of the tube (this can be autoclaved if necessary).
- Transfer the gel slice in this tube and freeze for 20 minutes at -20 °C.
- Place this tube in a 1,5 ml one and wait until the gel slice is defrosted.
- centrifuge 1 minute at maximum speed.
- The liquid collected in the 1,5 ml tube is the electrophoresis buffer with about 90 % of the PCR band.
- Determine the volume and add 2,6 volumes (95 % ethanol + 0,12 M NaAc), mix and put at roomtemperature for 15 minutes.
- centrifuge 15 minutes at 14.000 rpm (4 °C).
- remove supernatans (pellet is invisible) and add 125 µl 70% ethanol, mix gently and centrifuge 5 minutes at 14.000 rpm (4 °C).
- remove supernatans (pellet is invisible).
- Add destilled water (for approximately the same concentration as in the PCR mixture, add the same volume as you used for loading the gel).
back to homepage
